What Does the Catalytic Converter Do?
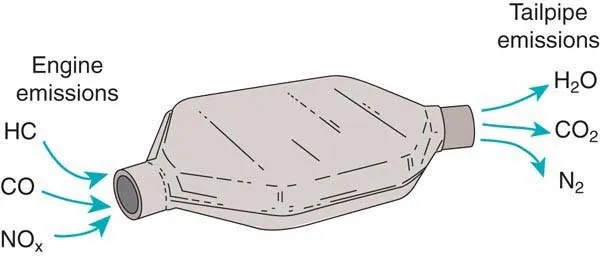
The catalytic converter uses a chamber called catalyst to convert harmful compounds emitted by the engine into safe gases, such as steam. Its function is to decompose unsafe molecules in the gas generated by cars before releasing them into the air.
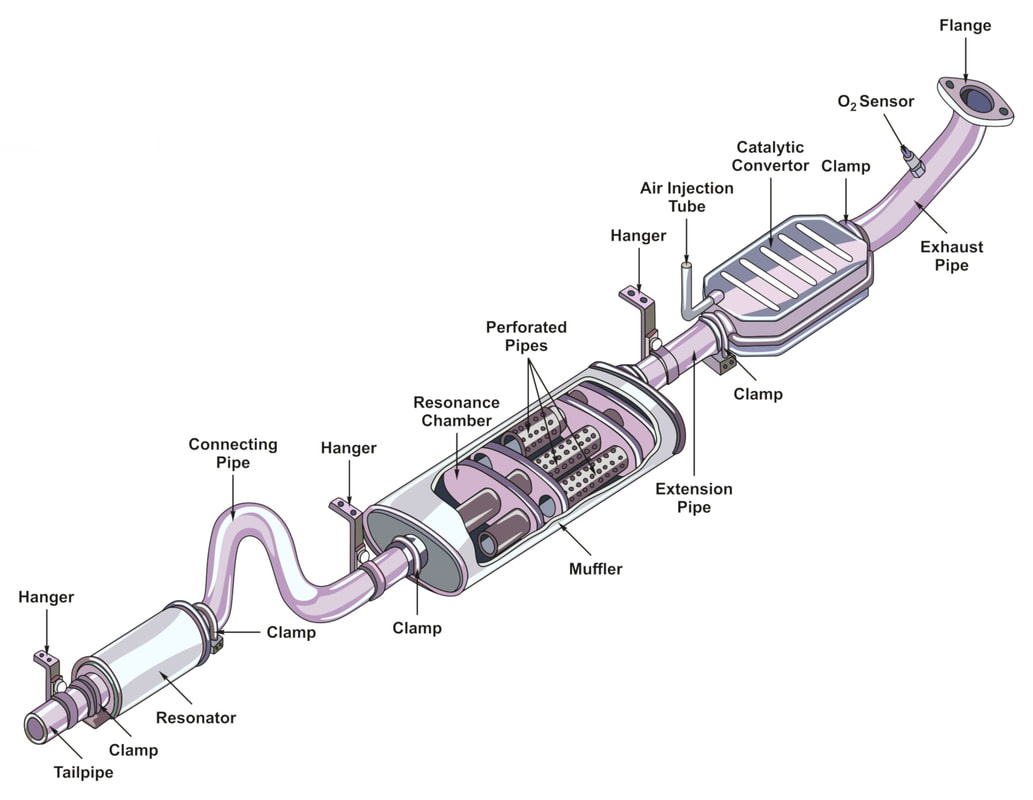
The catalytic converter is located at the bottom of the vehicle and looks like a large metal box. There are two pipes coming out of it. The converter uses these two pipes and catalysts in the process of safe gas discharge.
The gas is introduced from the “input” pipe connected to the vehicle engine. These gases are blown onto the catalyst, causing chemical reaction to decompose pollutants. The gas with less harmful gas now passes through the second pipe, which is the “output pipe” connected to the automobile exhaust pipe.
What Is Inside a Catalytic Converter?
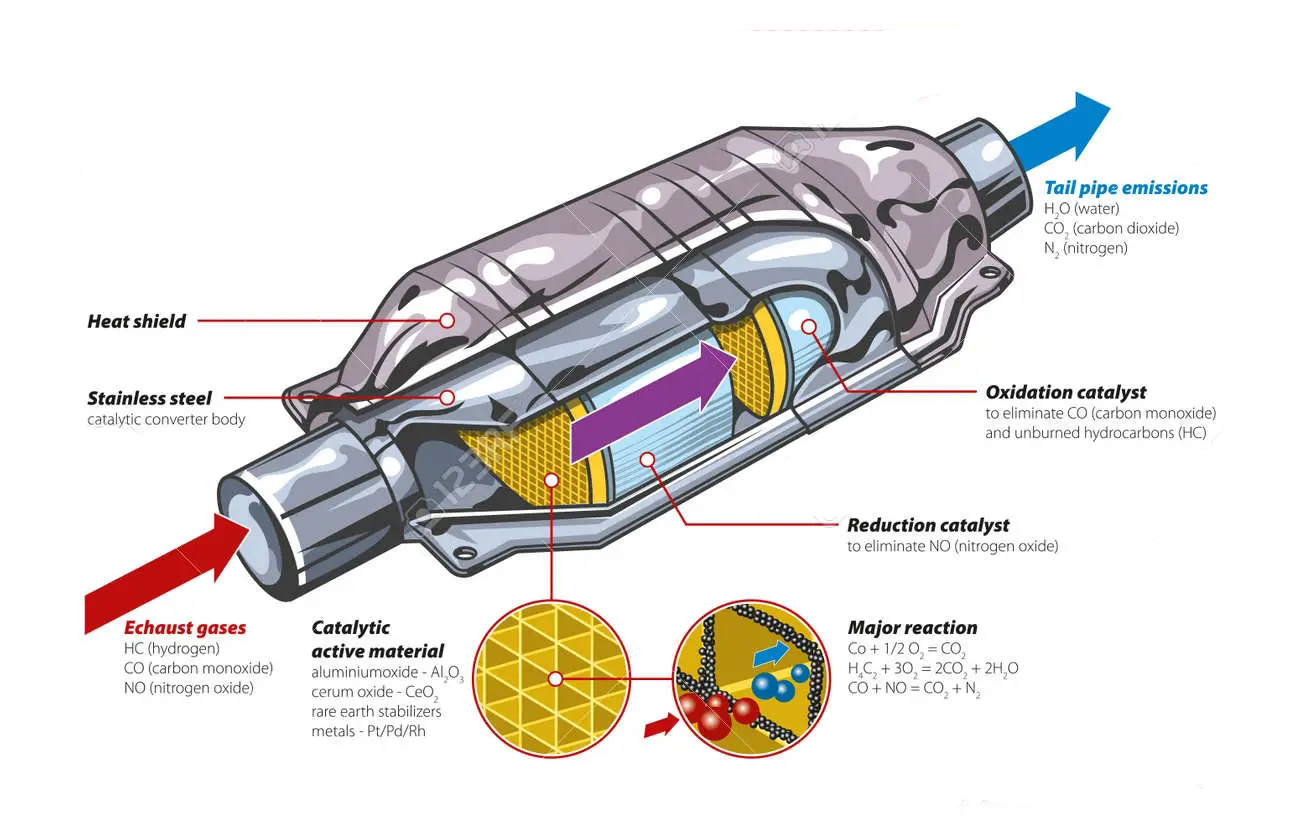
So what is the catalytic converter made of? The catalyst in the catalytic converter is usually made of platinum or similar metals such as rhodium or palladium. The gas flows through the ceramic honeycomb structure located in the cat shell. This is lined with metal with specific jobs, which play a role in reducing emissions. Two main types of catalysts may be used in automobiles:
Reduction catalyst: help reduce nitrogen oxide pollution by removing oxygen. Nitrogen oxides are decomposed into nitrogen and oxygen, which are harmless in themselves.
Oxidation catalyst: used to convert carbon monoxide into carbon dioxide through the reverse process of adding oxygen.
There is also an oxygen (O2) sensor near the catalytic converter, which can tell the electronic control unit (ECU) of the car how much oxygen is in the exhaust gas. This helps the vehicle to operate at a more efficient air-fuel ratio, thus enabling the engine to provide sufficient oxygen for the converter to complete the oxidation process.
Types of Catalytic Converters
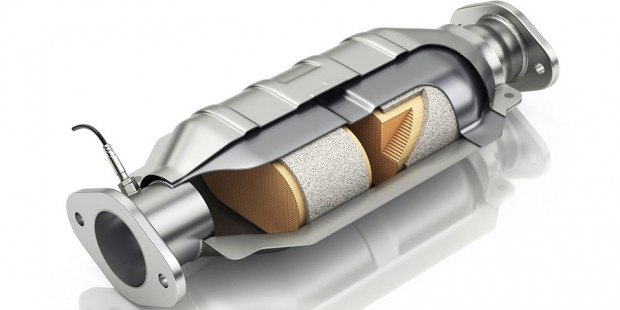
As mentioned earlier, there are two main catalysts – reduction and oxidation – that can be used to treat specific gases in the exhaust system.
Depending on the year of the vehicle and the type of catalytic converter, there may be no reduction catalyst. There are two main types of catalytic converters:
Two-way: It was not until 1981 that the bidirectional catalytic converter appeared on vehicles in the United States. They only have oxidation catalysts, which help to convert carbon monoxide into carbon dioxide. Hydrocarbons (unburned and partially burned fuel) are converted into carbon dioxide and water.
Three-way: Three-way catalytic converter has been used since 1981. This is the same as the performance of binary converter with reduction catalyst. As mentioned earlier, this is used to convert nitrogen oxides into nitrogen and oxygen.
The diesel engine uses a two-way catalyst, and the converter is also specially designed for diesel exhaust. The converters of these types of engines try to target particulate matter of soluble organic fractions. These are made of hydrocarbons combined with soot.
Post time: Mar-20-2023